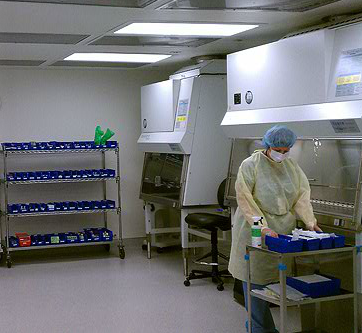
Our Services
LabMetrics, Inc. offers a number of services, which are beneficial to our Pharmacy and Laboratory customers. The following is a list of services provided by LabMetrics, Inc. Services are not limited to this list.
The following is a list of services provided by LabMetrics, Inc. Services are not limited to this list.
Biological Safety Cabinet Certification/Service and Repair (NSF/ANSI 49)
Laminar Flow Clean Bench Certification/Service and Repair (Different Standards May Apply)
Fume Hood Certification/Service and Repair (Different Standards May Apply)
Glovebox Isolator Certification/Service and Repair (CETA Standard)
Incubator Certification/Service and Repair (Manufacturers Specifications)
Centrifuge Certification/Service and Repair (Manufacturers Specifications)
Refrigerator Certification
Freezer Certification
Thermometer Calibration/Service and Repair (NIST Traceable)
Biological Decontamination (NSF/ANSI 49 Standard/ Chlorine Dioxide)
USP <797> Validation/Consultation
USP <800> Validation/Consultation
Cleanroom Certification/Service and Repair (Different Standards May Apply)
Testing includes, but is not limited to:
- airflow analysis
- particle count analysis
- microbial/fungal analysis (Air and Surface Samples)
- HEPA filter integrity testing
Monthly Microbial/fungal testing (A well developed environmental monitoring program is a proactive way to assure the cleanliness of the cleanroom complex, monthly monitoring is a great way of incorporating trending data to ensure the performance of your cleanroom)
Smoke Study/Smoke Pattern Test (This study plays a very significant role in the qualification and monitoring of the aseptic area ensuring your cleanroom’s airflow meets FDA expectations. This has been a regulatory requirement USP <797> for over 19 years intended to demonstrate unidirectional airflow exiting HEPA and sweeping action over and away from the product under dynamic condition. The overall objective of this study is that the particle will be driven from the HEPA, over the product on equipment, the personnel involved, the cleanroom floor, and finally the room raisers-the place where the particles exit the room.) We perform the test using the CRF4, which is the most innovative instrument available. It supports cleanroom goals described in industry standards such as ISO 14644-3 Annex B7, USP <797> Institute Airflow Analysis and Federal Standards 209E.

Terminal cleaning of Pharmacies, Laboratories, and equipment. This service includes a three-solution cleaning process (Bleach, Opti-Cide, 70% alcohol)
- thorough cleaning of ceilings, walls, and floors
- we move all equipment to thoroughly clean underneath and behind it.
- complete wipe down of BSC’s, laminar flow units, incubators, centrifuges, etc.
This service offers our clients an advantage by having thorough cleaning performed by our professional cleaners who have been trained in working in the cleanroom environment. Environmental cleaning is essential to preventing the spread of infection and eliminating environmental contamination. We have found that our customers are benefiting from weekly or monthly cleaning programs designed to drastically reduce the microbial/fungal burden in the cleanroom. By having terminal cleaning performed on a regular basis, you are helping to create a cleaner and safer working environment. This service also benefits the equipment, hoods draw air into them, filter it, and then send it to your work surface. If the air that the hood is drawing is full of dust and particles it is going to load the HEPA filters at a much faster pace. A routine cleaning program will cut down on the number of particulates in the air therefore lessening the burden on the HEPA filtration.
Monthly Microbial/fungal testing (A well-developed environmental monitoring program is a proactive way to assure the cleanliness of the cleanroom complex, monthly monitoring is a great way of incorporating trending data to ensure the performance of your cleanroom)
Smoke Study/Smoke Pattern Test (This study plays a very significant role in the qualification and monitoring of the aseptic area ensuring your cleanroom’s airflow meets FDA expectations. This has been a regulatory requirement USP <797> for over 19 years intended to demonstrate unidirectional airflow exiting HEPA and sweeping action over and away from the product under dynamic condition. The overall objective of this study is that the particle will be driven from the HEPA, over the product on equipment, the personnel involved, the cleanroom floor, and finally the room raisers-the place where the particles exit the room.) We perform the test using the CRF4, which is the most innovative instrument available. It supports cleanroom goals described in industry standards such as ISO 14644-3 Annex B7, USP <797> Institute Airflow Analysis and Federal Standards 209E.
Hydrogen Peroxide mist decontamination
- this achieves a 6-log level of decontamination (Covid-19 is a 4-log level) with a 99.999% kill rate of germs.
- use of chemical indicators to ensure treatments are science based and proof that the treatment achieved a 6-log level of decontamination.
- one treatment will eliminate pathogens; however, we could work with you to create a comprehensive, pro-active plan with ongoing decontaminations to meet your specific needs and budget.
Terminal cleaning of Pharmacies, Laboratories, and equipment
- thorough cleaning of ceilings, walls, and floors
- we move all equipment to thoroughly clean underneath and behind it.
- complete wipe down of BSC’s, laminar flow units, incubators, centrifuges, etc.
This service offers our clients an advantage by having thorough cleaning performed by our professional cleaners who have been trained in working in the cleanroom environment. Environmental cleaning is essential to preventing the spread of infection and eliminating environmental contamination. We have found that our customers are benefiting from weekly or monthly cleaning programs designed to drastically reduce the microbial/fungal burden in the cleanroom. By having terminal cleaning performed on a regular basis, you are helping to create a cleaner and safer working environment. This service also benefits the equipment, hoods draw air into them, filter it, and then send it to your work surface. If the air that the hood is drawing is full of dust and particles it is going to load the HEPA filters at a much faster pace. A routine cleaning program will cut down on the number of particulates in the air therefore lessening the burden on the HEPA filtration.
Staff Training on proper Hood Usage.
Pre and Post Construction Cleaning in Laboratory and Pharmacy Environments
Hazardous/Non-Hazardous drug Swab Testing
Drug Sterility Testing
Complete design and construction of cleanroom environments
Environments monitoring with in-house laboratory services
Active Air Sampling (microbial, fungal, asbestos, lead, arsenic, mercury, concrete, dust, pollen, PCB’s, VOC’s, insects, and many more)
Training options for staff on how to safely operate equipment and minimize user error and damage to equipment.
LabMetrics, Inc. also has our own certified cleanroom used for hazardous/nonhazardous sterility testing and a variety of other testing and uses to benefit our customers.
Surface Sampling (microbial, fungal, asbestos, lead, arsenic, mercury, concrete, dust, pollen, PCB’s, VOC’s, insects, and many more)
We provide our customers with the most up to date testing/cleaning techniques. All our test procedures comply with industry standards and our staff includes certifiers accredited by NSF International. We are on call 24 hours a day/7 day a week for any emergencies or issues our customers may have. We are dedicated in providing the best service possible to our customers and we are always available to answer any questions.